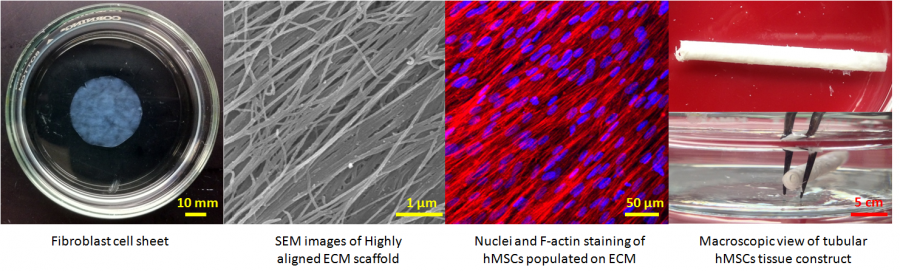
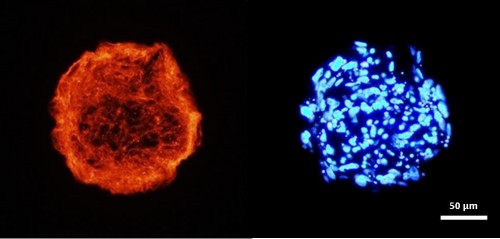
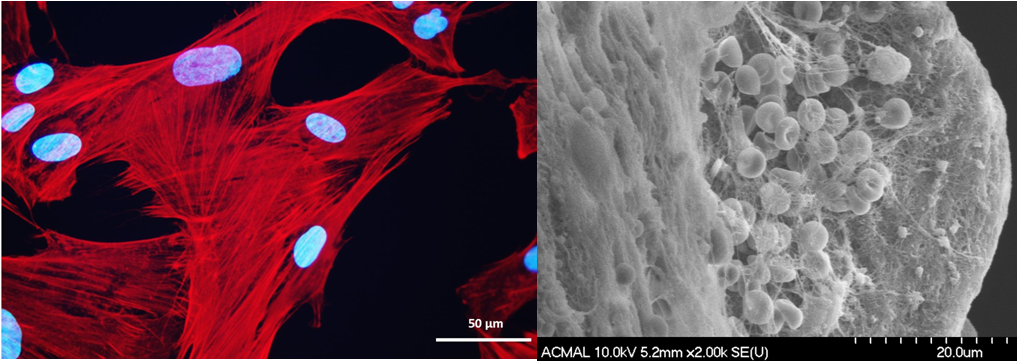
Engineering Completely Biological Small-Diameter Blood Vessel with Human Mesenchymal Stem Cell Sheets
Vascular grafts are in great demand because coronary artery diseases cause 12 million deaths in the world each year and account for half of all deaths in the United States. Despite the successful replacement of large-diameter blood vessels with non-biodegradable polymeric materials, critical issues remain in the creation of coronary heart disease-related small vascular grafts. So far no biomaterials and cell-based tissue-engineered blood vessel can meet the urgent needs of patients for coronary artery substitutes. Human mesenchymal stem cells (hMSCs) 1) are immunomodulatory and antithrombogenic, 2) can recruit endothelial cells and smooth muscle cells in vivo, 3) can be easily obtained and are highly expandable, 4) can secrete a variety of “trophic factors” (growth factors, cytokines, and adhesion molecules) to stimulate the regeneration of dysfunctional tissues. These unique properties confer hMSCs superiority for the fabrication of a tissue-engineered blood vessel with a single cell type. The objective of this project is to develop a cell sheet engineering strategy potentially suitable for the production of a completely biological and mechanically strong small-diameter vascular graft by manipulating hMSCs with biomimetic microenvironmental parameters.
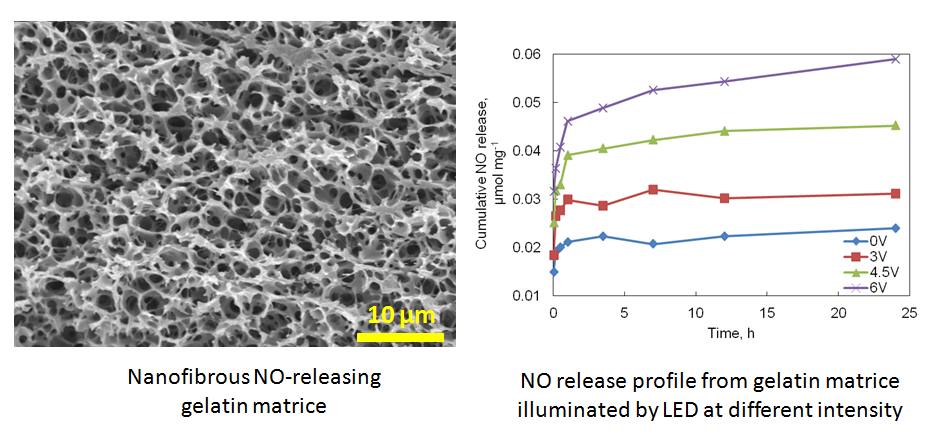
Nitric Oxide (NO)-Releasing Biomaterials for Vascular Tissue Engineering
Nitric Oxide (NO) is a very important signaling molecule involved in a variety of cellular events such as cell proliferation, adhesion and migration. NO also has multiple physiological functions such as protecting against invading pathogens, preventing platelet adhesion, improving angiogenesis, and inhibiting bacterial growth. We seek to functionalize the naturally derived protein scaffolds by conjugating NO donors and explore their biological applications in tissue engineering and regenerative medicine. The resulting materials are controlled to release NO at a physiological relevant level, which changes the organization of cytoskeleton and focal adhesion molecules. The NO-releasing natural biomaterials are currently under investigation with the purpose to improve their biological performance as an anticoagulant scaffold for vascular tissue engineering.
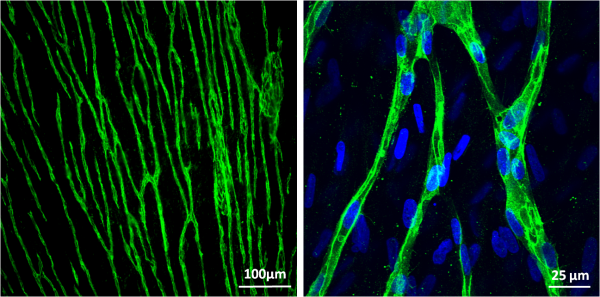
Prevascularization of hMSC Cell Sheets for 3D Prevascularized Tissue Fabrication
Despite the significant progress in tissue engineering in the past decade, one of the most critical obstacles hindering the implantation of large 3D engineered tissues larger than 100 µm is the inefficient mass transport of oxygen, nutrients, and wastes that can lead to necrosis. The preconstruction of capillaries with vascular or vascular-inducing cells is a reliable approach in supporting the in vivo tissue survival and accelerating the anastomosis of the preformed vasculature with the native blood vessel network. Compared with other cell types, hMSCs are more clinically relevant, and easier to obtain and expand. In addition to functioning as pericytes, hMSCs can potentially differentiate into desired cells or secrete necessary growth factors and cytokines as required by the regenerating tissue. Therefore, the dual functions that hMSCs can play make them more attractive than other cell types in engineering prevascularized complex tissues. Our approach is to make prevascularized hMSC sheets, which will then be used as building blocks to construct prevascularized 3D tissues. While only a very small number of hMSCs are committed to play the role of mural cells during the in vitro vasculature formation, the majority of them constitutes the cell sheet and contributes in tissues regeneration upon implantation in vivo in the next step.
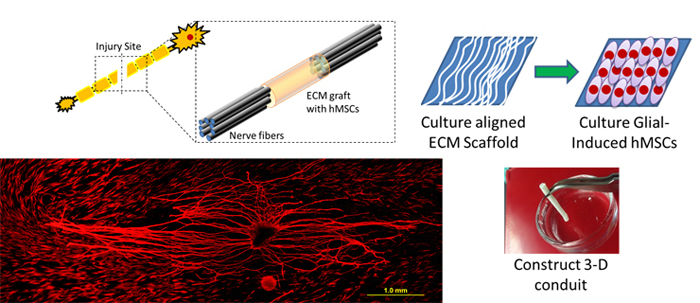
Neural Tissue Engineering with hMSCs and Natural Nanofibrous ECM Scaffold
Millions of car accidents, sports injuries and violence crimes lead to traumatic damage, causing various levels of neural injury to the victims. The current treatments with tissue engineered grafts are not ideal for large lesions between two ends of the transected nerves, and harvesting autologous nerve grafting is not always feasible for every patient. hMSCs have the ability to maintain multipotency while self-renewing, suppress immune response, and serve as vascular pericytes. Importantly, hMSCs have the ability to differentiate into glial and neural specific protein expressing cells. The objective of this project is to develop a completely biological neural conduit by manipulating glial-differentiated hMSCs and a 3D aligned nanofibrous ECM scaffold. We hypothesize that the neural conduit combined by glial induced hMSCs and ECM scaffold will not only provide a mechanical supporting platform, but also serve as a growth factor and cytokine reservoir for transected nerve repair.
Engineering hMSC Spheroids for Lymphedema Treatments
Female breast carcinoma is the most prevalent cancer worldwide. Many of these patients require the removal of some or all of the breast tissue. During this surgery it is common to also remove the nearby axillary lymph nodes and surrounding lymph tissue to prevent recurrence and metastases. Unfortunately, the removal of this lymph tissue leads to secondary lymphedema in 26% of the cases. This lymphedema can be very painful and leads to subsequent skin problems. There are currently no treatments for lymphedema, with only palliative options available. A cellular therapy that can effectively reduce lymphedema and stimulate tissue regeneration is critically needed. We are exploring a preemptive cellular therapy by using hMSCs, which are well known for their ability to differentiate into multiple cellular linages and provide a milieu of trophic factors that can help to regenerate tissue. Their efficacy is further enhanced when these cells are grown in a spheroid form and under physiologically low oxygen condition that highly mimic their native three-dimensional (3D) environment. The hMSC spheroids have great potential to reduce scar formation and enhance lymphangiogenesis in the wound area post-axillary lymph node dissection, leading to a reduction in lymphedema occurrence.
Immunoregulatory Property of hMSC Cell Sheets
hMSCs are immunoregulatory and immune-privileged; therefore, shows great potential in overcoming the application limitations of non-autologous cells in cell therapy and tissue engineering. Cell sheets grown from hMSCs offer tremendous opportunities for tissue repair and reconstruction. Our previous studies showed that hMSCs cultured on nanopatterned substrate and physiological oxygen conditions create a confluent, well-aligned cell layer with well-preserved multilineage differentiation ability and more ECM proteins. The objective of this project focuses on investigation of the immunoregulatory property of hMSC cell sheets obtained from various culture conditions.
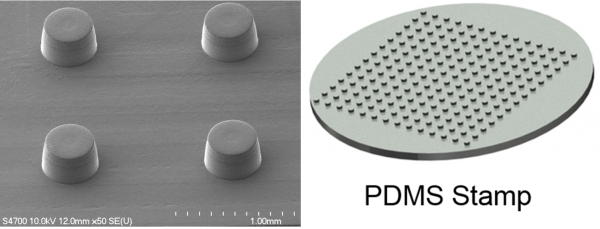
Micro-fabrication of Cell Sheet Fragments
Micro-sized cell sheet fragments are desired in non-invasive regeneration therapies such as injection. We are developing micro-patterns by using photolithography for the construction of cell sheet fragments with controllable size. The cell sheet fragments are detached from thermo-responsive polymer coated micro-patterns under a temperature lower than 32°C. The cell sheet fragments will be applied in injectable therapies.
Development of Metal Organic Frameworks-Coated Gelatin Microspheres
Zeolitic imidazolate framework ZIF-8 is a highly porous metal-organic framework material with excellent thermal and chemical stability. These unique properties make ZIF-8 promising for drug delivery application. Gelatin is a denatured collagen that has been extensively employed as a biomaterial. Our research focuses on the synthesis of ZIF-8 coated gelatin hydrogel microparticles for drug delivery and tissue engineering applications.
Cytotoxicity Examination of Bioabsorbable Stent
Long term stenting of the vasculature can lead to many problems, including chronic inflammation, late stage thrombosis and stent strut disruption. Researchers are now developing stents that can be gradually dissolved after they are no longer required. Researchers in Dr. Jaroslaw Drelich’s lab at the Department of Material Science Department of Michigan Tech are currently investigating Zn as a possible material for biodegradable stents. Collaborating with Dr. Drelich’s lab, we are examining, in vitro, the Zn cytotoxicity in a created microenvironment that mimics the context of arteries where the stent is placed in. Another material’s cytotoxic properties that is currently being investigated is zeolitic imidazolate framework (ZIF), which has potential applications in drug delivery and tissue engineering scaffolds.